
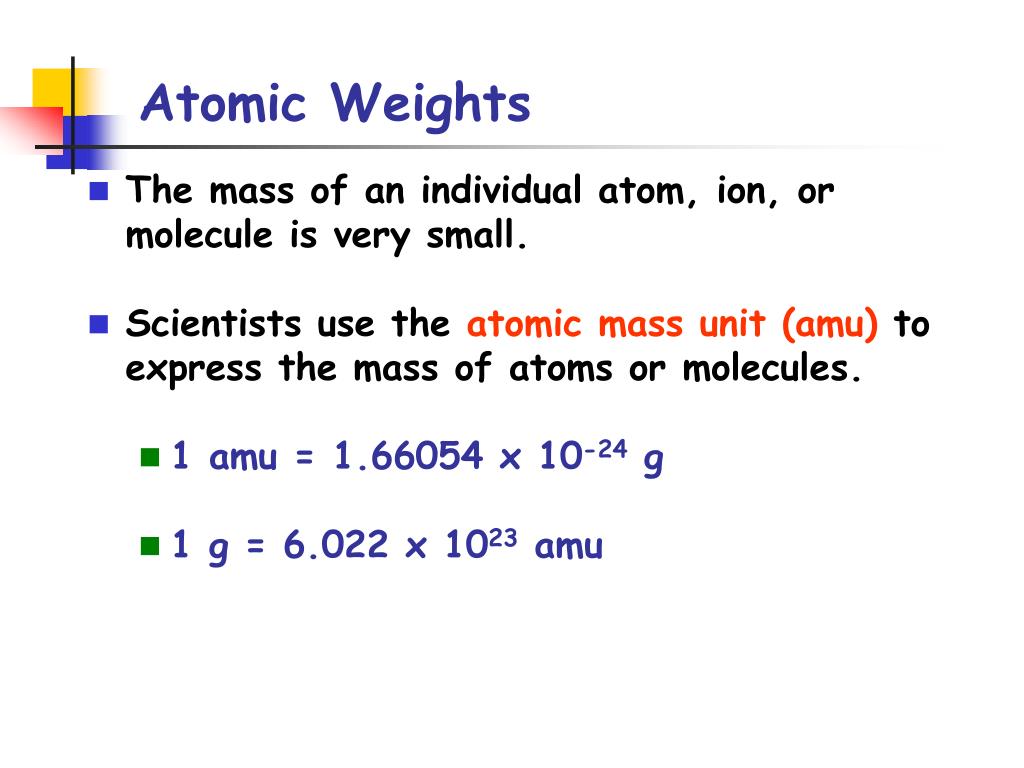
Atomic Weights and Isotopic Compositions (version 4.1), 2015, National Institute of Standards and Technology, Gaithersburg, MD, accessed November 2016. When an organism dies, it stops taking in carbon-14, so the ratio of carbon-14 to carbon-12 in its remains, such as fossilized bones, will decline as carbon-14 decays gradually to nitrogen-14 2 ^2 2 squared. Relative atomic mass The mass of an atom relative to that of carbon-12. As animals eat the plants, or eat other animals that ate plants, the concentrations of carbon-14 in their bodies will also match the atmospheric concentration. As plants pull carbon dioxide from the air to make sugars, the relative amount of carbon-14 in their tissues will be equal to the concentration of carbon-14 in the atmosphere. Though individual atoms always have an integer number of atomic. No single carbon atom has a mass of 12.01 amu, but in a handful of C atoms the average mass of the carbon atoms is. These forms of carbon are found in the atmosphere in relatively constant proportions, with carbon-12 as the major form at about 99%, carbon-13 as a minor form at about 1%, and carbon-14 present only in tiny amounts 1 ^1 1 start superscript, 1, end superscript. The standard atomic weight is the average mass of an element in atomic mass units (amu). This is the average atomic mass of carbon. See original paper for the range of these elements from different sources Isotope-abundance variations and atomic weights of selected elements: 2016 (IUPAC Technical Report), Pure Appl. For example, carbon is normally present in the atmosphere in the form of gases like carbon dioxide, and it exists in three isotopic forms: carbon-12 and carbon-13, which are stable, and carbon-14, which is radioactive. List of Elements with Range of Atomic Weights.


 0 kommentar(er)
0 kommentar(er)
